

Did you know that all the light bulbs in your house and the screen of your smartphone are made of LEDs?
The above pictures are of LED lights. These lights are used in most areas today. LEDs are used in everything from home lighting to decorative lighting. Today’s televisions, smartphones, and monitors are also equipped with LEDs. So today we’ll talk about how these LEDs work, why they’re so popular, and how they differ from regular diodes. Let’s start.
What are LEDs?
LED is an abbreviation for light-emitting diode. These are semiconductor devices that emit light when an electric current flows through them. It produces light by recombination of electron-hole pairs. LEDs are similar to regular diodes. LEDs are ON when forward-biased only.
In 1962, Nick Holonyak Jr. invented the first LEDs that emit visible light.
How does LEDs works??
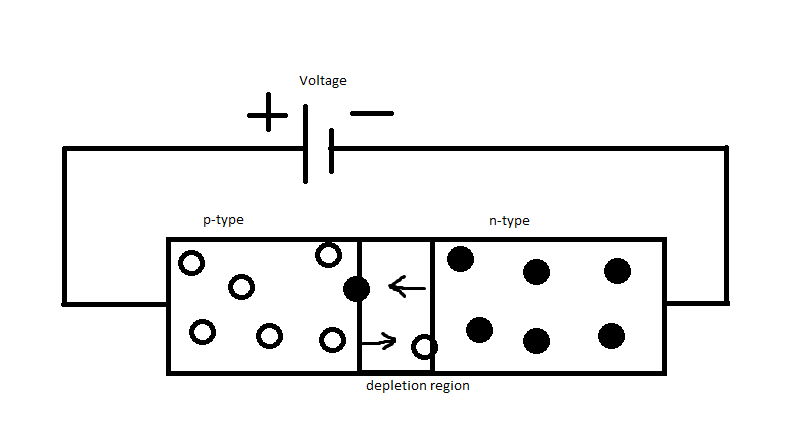
The operating principle of LEDs is the same as the operating principle of diodes. LEDs are composed of p-type and n-type semiconductors and are connected together, as shown in the figure. A p-type semiconductor has three electrons in its valence shell, and an n-type semiconductor has five electrons in its valence shell. Holes are the majority charge carriers in P-type semiconductors, and electrons are the majority charge carriers in N-type semiconductors. A depletion layer is formed between the n-type semiconductor and the p-type semiconductor. Under normal conditions (when no voltage is applied to the PN junction), the depletion region creates a potential that prevents electrons from diffusing from the n-type semiconductor to the p-type semiconductor.
An atom is stable when its valence shell has four or eight electrons. To be stable, n-type semiconductors try to donate one electron from their valence shell, and p-type semiconductors try to accept one electron to become stable.
When a forward bias voltage greater than the potential barrier is applied to the PN junction, the cathode of the battery repels the free electrons contained in the valance shell of the n-type semiconductor towards the p-type semiconductor. When the applied voltage is larger than the potential barrier, the electrons pass through the depletion layer and recombine with the holes in the p-type semiconductor. During the recombination, the electron loses some energy.
In a normal diode, this energy is in the form of heat or other invisible forms of light. But with LEDs, these energies are in the form of visible light. This process continues until a forward bias voltage is applied to the PN junction.
How do LEDs emit different colours of light?
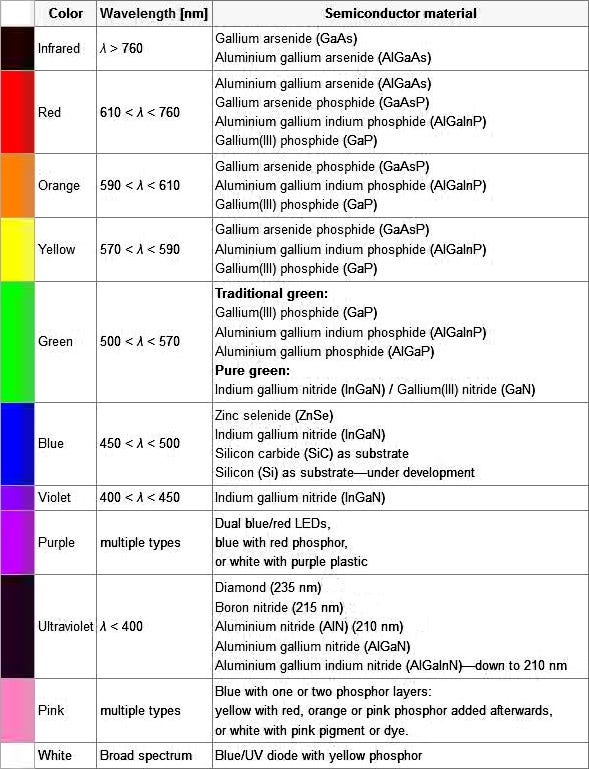
It depends on the materials used to make the semiconductor. All materials emit energy in the form of photons (light particles) of a particular wavelength. The wavelength of the photon determines the colour of the light. This table shows the materials used to create different colours of light.
Why LEDs are so famous?
Because they are:
· Energy efficient
· Has high brightness
· Small in size
· Variety of colours of light
· Less energy is wasted into heat
· More durable etc.


0 Comments